JAR OF HOPE FOUNDATION BRINGS SICK YOUNGSTER TO UNITED STATES FOR TREATMENT
It’s not easy to get a little Irish boy with a fatal illness to the United States for medical treatment, especially during COVID-19. But JAR of Hope has done it.
Alfie Pentony, 7, lives in Northern Ireland with his parents, Colleen and Jamie, and two brothers, according to a press release from JAR of Hope.
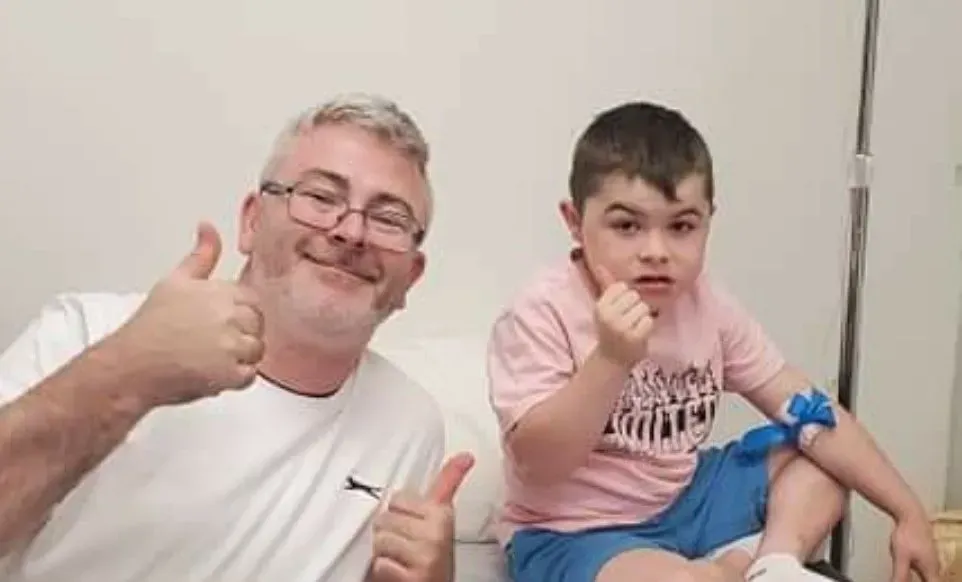
At the age of 4, Alfie was diagnosed with a very rare, always-fatal muscle-wasting disease called Duchenne Muscular Dystrophy. By their early teens, victims of Duchenne are in wheelchairs and by their early 20’s they are in graves after suffocating to death, according to the press release.
Alfie is participating in a year-long study called Operation Lifeline, conducted by the Salerno Center for Complementary Medicine in New York City, and sponsored by the JAR of Hope foundation of West Long Branch, which raises funds to research a cure for Duchenne.
Because of COVID-19, Alfie and his dad were initially denied entry into the United States for this month’s tests, according to the press release.
“This is a very promising study and we were determined to get Alfie here for the next phase, which is this week,” said Jim Raffone of JAR of Hope, who, with wife Karen, founded the organization in 2014 after their 4-year-old son James Anthony was diagnosed with Duchenne, according to the press release.
“So I began hitting the phone, to Congressman Chris Smith of New Jersey; Tom Lantos of Congress’ Human Rights Commission; Dr. John Salerno of the Salerno Center; and Patrick Smith, legal counsel to Congress’ Human Rights Commission, who was able to connect us to the U.S. State Department and the American Embassy in Ireland,” Raffone said.
In Operation Lifeline, four boys with Duchenne receive infusions of a chemical compound called JAR914 every three months and their muscle strength is measured before and after the treatment. The results have been very encouraging, according to the press release.
“We had brought Alfie here every three months for the treatments, since last October,” Alfie’s dad Jamie said. “But to get here now we needed assistance and JAR of Hope was really there for us. I don’t think we could have gotten Alfie here without them.”
“This is a ground-breaking study,” Raffone said. “For the first time, we are seeing results that may actually prolong the lives of kids with Duchenne … and maybe even lead to a cure. So when Alfie’s parents contacted us, we vowed we would not stop until we got him here.”
Finally, only weeks before this week’s phase of Operation Lifeline, Jamie Pentony and Alfie received permission to come to the United States and Alfie is undergoing the infusions right now, according to the press release.











